Separate databases allow for differences across countries such as language, different patient pathways, different units of measurement, however it will require additional work in ensuring consistant data collection between databases to allow for data to be merged for a single trial analysis.
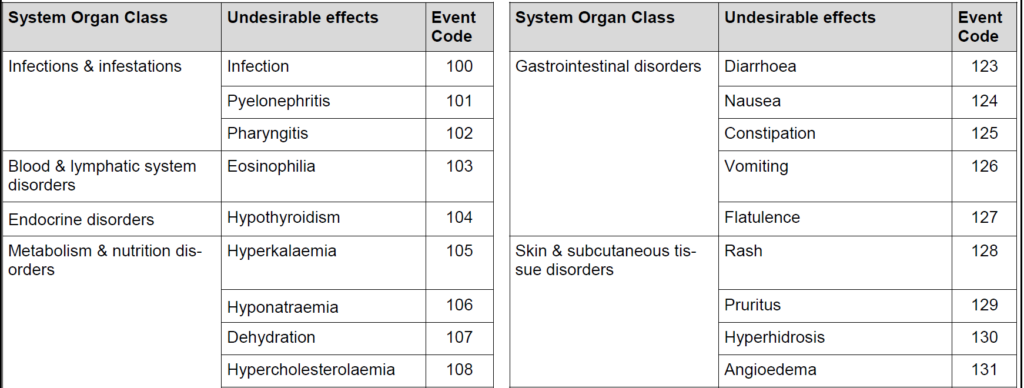
- Avoid questions that are answered with free text. Free text answers are never easy to deal with and language or terminology difference. If drop down or yes/no questions are not feasible consider ‘code lists’, where a numerical or alpha numeric code is used instead of the full name / free text.
Example code lists:

- If the answers have a choice of answers make sure the answers are mutually exclusive and the list is exhaustive (unless you include an ‘other’ category).
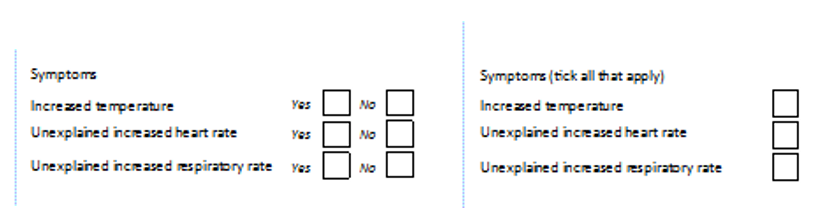
- If more than one option can be selected, consider a yes/no option for each option:
- E.g. if participants can experience more than one symptom consider a yes/no for each symptom. This is easier to validate on the database, and also you can be sure that symptoms have not been missed by accident.
In the second example below, an empty box could be a genuine ‘no’ or could mean that the box had been skipped.
- Avoid ambiguous questions.
- Consider if Data Protection laws are different or have different requirements, or even the same law (e.g. GDPR) may have different interpretations locally.
- Consider legislation around data sharing required as part of the trial, or potential future data sharing
- Are the data items available in overseas centres? It shouldn’t be assumed that data items that are ‘standard’ in the UK will be ‘standard’ in other countries.
- Does te data collection rely on a particular patient pathway? Differences in healthcare provision may affect this.
- Is follow-up data required after discharge from the index operation, or data not collected by the recruiting centre? Out of hospital health care provision may be very different in other countries.
- Does the data collection allow for differences in standard practice? Is terminology standardised or have you allowed for local variations?
- Use local experts where possible to check the above.
- Data acquisition – how will this be achieved?
- Where should database be hosted?
- How will data be retrieved from the separate databases, and how will data be merged/mapped?
- Robustness and support – especially if used over very diverse and distant network with disparate platforms.
- Availability of the local network if the local databases are internet based – may necessitate complex ‘off-line’ solutions that can sync themselves. Plus, internet transfer may be needed for transfer of data to coordination centre.
- Data security – especially if any offline data collection is involved. This may be more difficult to control with separate databases.
- Data integrity – especially if any offline data collection is involved (i.e. ensuring data is not lost/changed without audit record). More problematic with separate databases.
- IT support may be required in multiple languages and available 24 hours to allow for different time zones. Will this be provided locally if using separate databases?
- Central user management will probably not be possible.
- User acceptability testing may need to be coordinated locally.
- Differences in time zone may cause issues e.g. processes maybe be scheduled to run overnight UK time, and slow the system down when other countries want access
Language issues are less of an issue if a local database is used. However, translation of any transferred data may be needed.
- Do CRFs (paper or electronic) need translation (and back translation)?
- Are there any PROMs? Does this need translation? If they are validated instruments, are they available in other languages? If not, the authors may not authorise their use in another language.
- Use of SMS text messages (e.g. questionnaire reminders, or even simple questionnaires via text) may not be possible especially if they need to be translated into local languages.
- Cultural barriers
- Is there an agreed ‘working language’?
- Encouraging participating sites to do a ‘dry run’ with CRFs to identify any potential problems is a useful tool. If this isn’t done, use the SIV to go through the CRFs with the site to identify
issues. This may only need to be done once per country depending on the degree of intra-country variability (however don’t trust clinicians who say ‘everyone does it like this here’!)
- User training requirements.
- Managing DEQCs (data entry quality check – where a random selection of CRFs are checked against the database to check for data entry mistakes that would not be picked up by inbuilt validation checks) and DCFs (data clarification forms) will both be more challenging.
- Units of measurement and standard ranges may have more variability than a UK based trial. Can the database accept/cope with different units?
- Health economics - even feasible in international studies?
- How is any non-data information be collected? E.g. scans, audiorecordings, videos, photos